1. Introduction
2. Experimental
2.1 Reactor
2.2 Materials
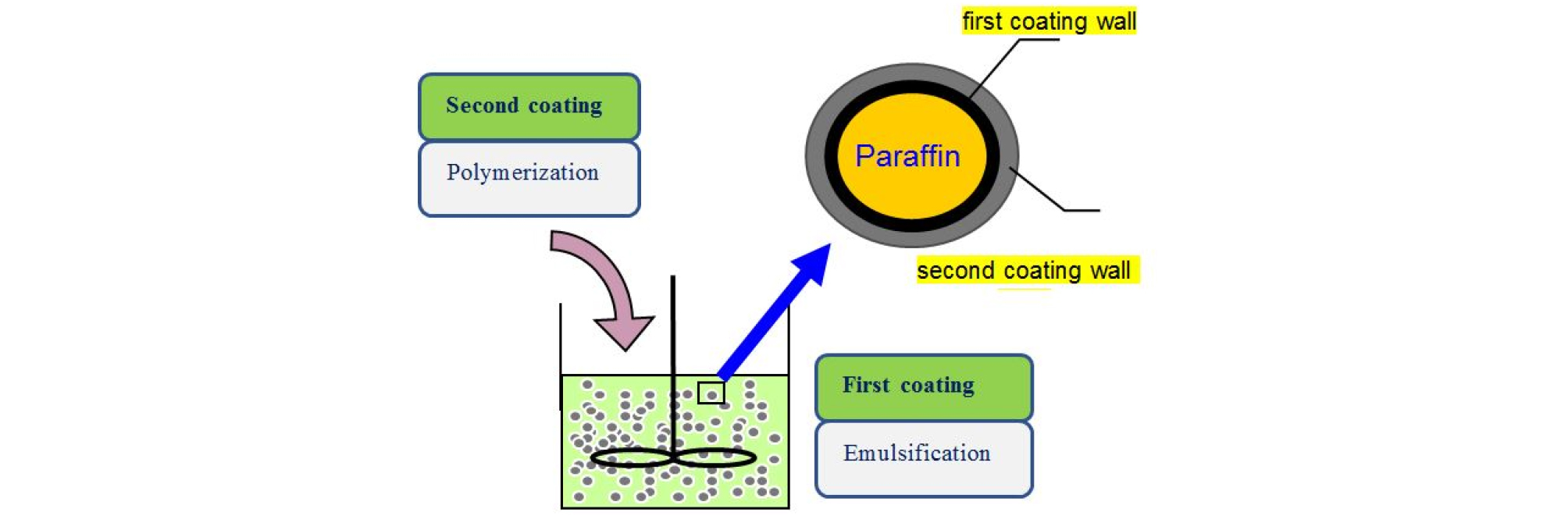
2.3 Encapsulation method
2.4 Physical properties of encapsulated particles
3. Results
3.1 Emulsifier selection
3.2 First encapsulation method
3.3 Second Encapsulation
3.4 Physical properties of encapsulated particles
4. Discussion
5. Conclusion
1. Introduction
Latent heat is the heat that a material absorbs or releases when it transitions from solid to liquid, or liquid to solid. Since each phase has a different phase transition temperature, it can absorb and store energy at various temperatures. Latent heat storage is the highly efficient method of storing thermal energy. Phase change material (PCM) is one of the most efficient energy storage material1). The two common groups of PCM are organic and inorganic materials2). Organic and inorganic materials are two most common groups of PCMs34). Organic materials can be described as paraffin and non-paraffin. Most of organic material PCMs are non-corrosive and chemically non-corrosive and chemically stable with little or no sub-cooling3). However, organic materials are reported as low thermal conductivities, high changes in volume on phase change and flammability345). In contrast, inorganic materials such as salt hydrate and metallic have a high latent heat per unit volume, high thermal conductivities and non-flammable. However, they are corrosive to most metals and suffer from decomposition and sub-cooling, which can affect their phase change properties456).
To solve problems of pure PCMs, microencapsulation technology has been developed and successfully used in wide range of applications78). Microencapsulation is a process of forming a shell around micronized materials (both liquids and solids), which range in size from less than 1 mm to more than 300 mm9). The advantages of microencapsulated paraffin wax are (1) reduce the reactivity of the paraffin wax with the outside environment, (2) increase heat-transfer area, and (3) permit the core material to withstand frequent changes in volume of the storage material, as the phase change occurs. The two main shell materials used for micro-PCMs are melamine-formaldehyde (MF) and acrylic polymer. The MF shell is a very durable shell but it contains formaldehyde component, a probable human carcinogen. It can contact with skin through all bedding and apparel products that contain PCMs microcapsules. On the other hand, among organic PCM, paraffin is one of the most suitable materials for PCM because it has a large amount of latent heat, low harmfulness to human body, stable, no reactivity and various melting temperatures depending on the number of carbon atoms10). In recents reports, paraffins are encapsulated in several materials but there is no study on paraffin double-walled microencapsulation.
In this study, we have developed double-walled microencapsulation technology using acrylic polymer (with methyl methacrylic acid (MMA)) and melamine polymer for thermal energy storage at various melting temperature, which is most commonly used as a latent heat storage material in submicron size and investigate its effect. The melting temperature was in the range of 6°C ~ 70°C and the physical properties of encapsulated paraffin was tested using differential scanning calorimetry (DSC) and scanning electron microscopy (SEM) analysis method
2. Experimental
2.1 Reactor
The size of reactors used in this study was 0.5 L, 2 L, 10 L. The 0.5 and 2 L reactors were double jacketed to maintain the water at constant temperature. The stirrer on the upper part of the reactors can change the stirring speed from 200 to 500 rpm. The 10 L reactors maintain water temperature using heating mantle. The stirrer on the upper part of the reactors can change the stirring speed from 100 to 500 rpm. Samples can be collected easily from all reactors.
2.2 Materials
The paraffin encapsulation is microencapsulated using a double-walled technique. The polymers used for wall material are acrylic polymer and melamine polymer. The acrylic polymer was prepared by adding various monomers such as methyl acrylate and butyl methacrylate to MMA. MMA used as main material in acrylic polymer (Junsei Chemical Co.). Methyl methacrylate is also referred as α-methacrylate (C6H8O2, melting point -48.2°C, boiling point 101°C, specific gravity 0.9440 at 20°C). Ethylene glycol dimethacrylate (EGDMA) and divinyl benzene (DVB) were used as crosslinking agents (0-5.0 wt% of monomer was used)1112). Industrial melamine and formaldehyde were used as 1.5 to 3.0 mol/mol of melamine for crosslinking polymerization. The effect of initiator on polymerization was investigated using ammonium persulfate, potassium persulfate, t-butyl hydroperoxide and AIBN (2.2’-azobis isobutyronitrile). The amount of initiator is 1.0 ~ 4.0 wt% based on the monomer. In order to investigate the effect of microcapsule in physical properties, colloidal silica (S-Chemical Co. 40 wt%) and sodium nitrite (Dong Yang Chemical Co.) were used. Hydroquinone (Samchum Chemical Co., 99%), a polymerization terminator was prepared at 1 wt% to measure the conversion rate. HCl and NaOH 1N were prepared to control pH.
2.3 Encapsulation method
(1) Emulsifier selection
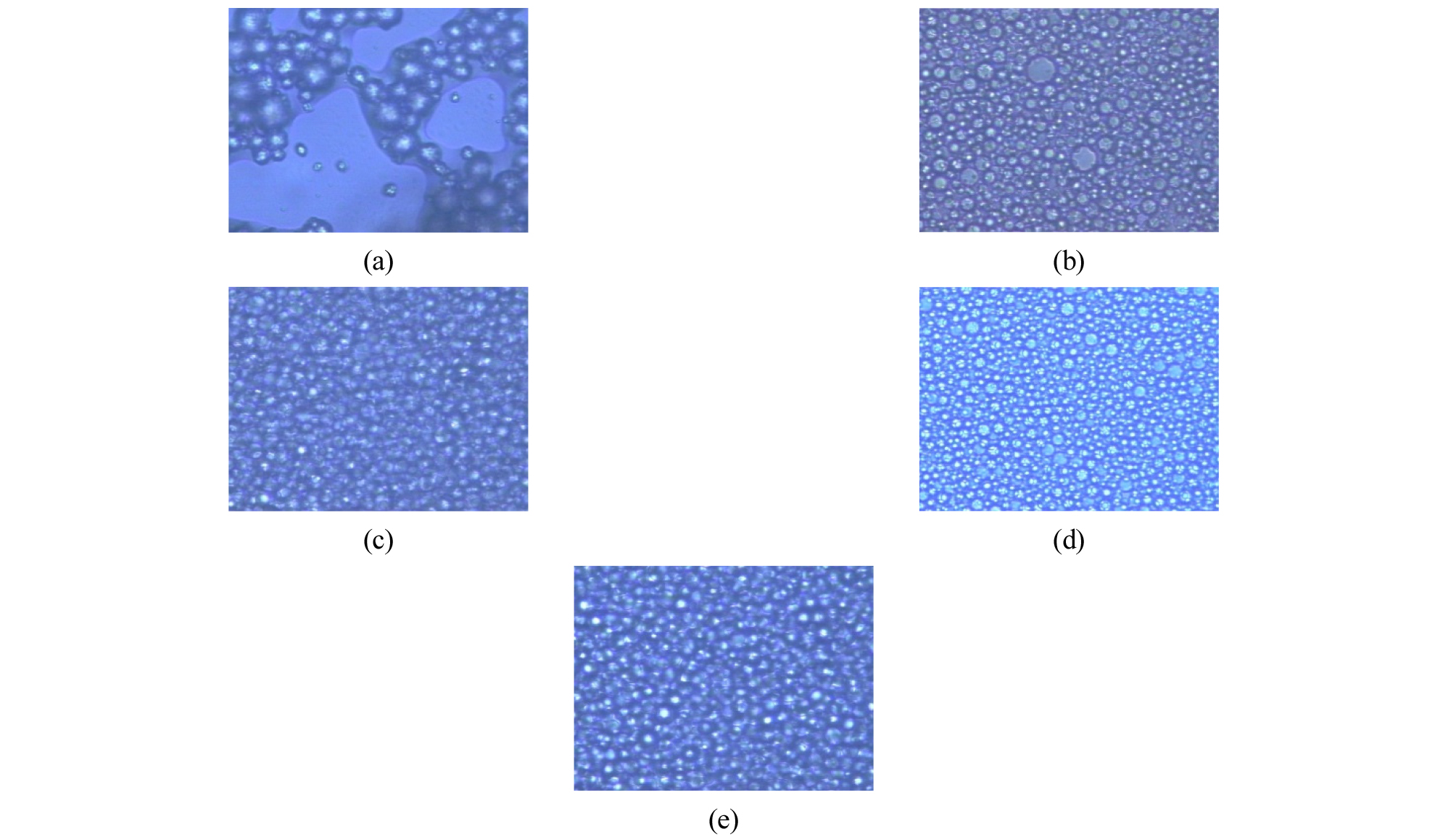
Emulsifiers play an important role in microencapsulation. It provides stability during paraffin emulsification and also affects the size of microcapsules. Therefore, in this study, the most suitable emulsifier was selected through the emulsion stability experiment according to the kind of emulsifier. The stability was tested with different emulsifying agents and emulsifying methods as below.
1)Add 133.3 ml Emulsol-P + 33.35 g PCM and homogenize at 8,000 rpm for 10 minutes (a)Add 16.705 g MMA + 11ml EO polymer emulsifier and homogenize at 8,000 rpm for 8 minutes (b)
2)Add 133 ml Emulsol-P + 33.69 g PCM + 16.7 g of MMA and homogenize at 8,000 rpm for 10 minutes (c) Add 11ml EO polymer emulsifier and homogenize at 8000 rpm for 10 minutes (d)
3)Add 133 ml Emulsol-P + 33.44 g PCM and 16.7 g MMA and homogenize at 8000 rpm for 10 minutes. Then add 11 ml of the EO polymer-based emulsifier and stir at 650 rpm for 10 minutes with a general stirrer (e)
(2) First encapsulation method
The polymerization is carried out by an emulsification step of dispersing the organic phase in water containing phase with an emulsifier. A starting step of polymerization was conducted by adding monomers and an initiator. Then an activating step of polymerization was realized by increasing the temperature to polymerization temperature. To investigate the optimal encapsulation reaction conditions, the effect of each condition was examined. Sodium peroxydisulfate, ammonium peroxydisulfate and sodium hydrogen sulfate monohydrat were used in various conditions to determine the optimum initiator.
Several ratios of PCM to monomer are investigated to obtain the stable capsulation. The ratios are 1 : 1 (PCM ratio is 50%), 2 : 1 (PCM ratio 67%), 3 : 1 (PCM ratio 75%), 4 : 1 (PCM ratio 80%) and 9 : 1 (PCM ratio 90%).
Without addition of the crosslinking agent, most of the powders obtained by encapsulating with only monomer MMA did not cause a serious problem when they were tested by SEM, temperature raising, solvent, etc. However, problems such as breakage of the capsules could occur regularly12)13). Thus, the effect of crosslinking agent was investigated using EGDMA and DVB were used as cross - linking agents and the amount 0.5 ~ 5.0 wt% of monomer was used11).
Polymerization temperature is a factor affecting the polymerization rate of monomer. It is known that the high temperature, the high polymerization rate and better monomer diffusion were reported14). In this study, polymerization temperature from 60 to 90°C was investigated.
When the amount of the initiator was changed, the effect on the encapsulation was not significant, but the encapsulation ratio of the PCM was greatly improved when the monomer feed rate was controlled. In order to control the monomer feed rate, the following experiment was carried out following these below steps:
1)Add a certain amount of emulsifier to 400 ml of water at 60°C and stir at 4,000 rpm for 5 minutes (solution 1).
2)Melt 100 g of paraffin by stirring it at 60°C (solution 2).
3)Mix solution 2 with solution 1 and emulsify by homogenizer for 10 minutes.
4)After adding an initiator, temperature in the polymerization vessel is raised to 80°C, the monomer is polymerized by injecting the monomer at a constant rate using a metering pump. After completing the injection, keep them for 1 hour to complete polymerization.
5)After lowering the temperature to room temperature, eliminate the solid by using a stainless-steel sieve.
(3) Second encapsulation
After the first encapsulation, a certain amount of melamine and 35% formaldehyde are added to proceed the second encapsulation15). Since the paraffin is encapsulated in the acrylic polymer by the primary encapsulation, there is no problem in the stability of the capsule when melamine and formaldehyde are added.
The amount of paraffin in the second encapsulation is determined according to the amount of the secondary encapsulated polymer. In this experiment, encapsulated reaction solution of primary encapsulation was changed from 21 to 36 g with 100 g of initial paraffin (Table 1).
Table 1 Change in content of paraffin in capsule according to melamine dosage (Initial paraffin 100 g, based on MMA 12 g)
| Melamine (g) | Calculated paraffin content (wt%) |
| 21 | 83 ± 2 |
| 24 | 80 ± 2 |
| 30 | 77 ± 2 |
| 36 | 73 ± 2 |
The physical properties of the capsules according to the drying method were confirmed. Drying was performed by spray drying, freezing drying, and drying in a thermostat, and the appearance of the obtained capsule powder was compared.
The paraffin double-wall microencapsulation method was presented in Fig. 1.
2.4 Physical properties of encapsulated particles
(1) Particle size and distribution
SEM was used to observed the size of the particles produced and the surface appearance of the particles. In the scanning electron microscope observation, the sample was treated with a carbon tape on the plate, a predetermined amount of sample powder was dropped and a platinum coating was performed under vacuum for 80 seconds. After the coated sample was mounted in high vacuum chamber, the particle size and surface state were observed.
(2) Identification of double capsules
The capsules made in this study are double capsules which are firstly encapsulated with acrylic polymer and secondarily encapsulated with melamine. To confirm this, a cross-sectional photograph of the capsule was taken. Only single-wall encapsulated particles and double encapsulated particles were dispersed and hardened in the epoxy resin, then cut into a single layer and measured with an electron microscope.
(3) Encapsulation efficiency
The encapsulation efficiency was determined using the ratio of encapsulated PCM to the initial injected PCM. After the polymerization, the temperature is lowered to room temperature, and the powder obtained through lyophilization and put into a test tube in a certain amount. Then add a certain amount of n-hexane as a solvent for PCM to the test tube and shake well. After removing n-hexane, add a certain amount of n-hexane and shake. Then weigh the remaining powder and analyze DSC (result 1). The n-hexane used above is analyzed by gas chromatography (GC) to analyze the contained PCM (result 2). Paraffin content was calculated after calculating the analysis results of (result 1) and (result 2) (result 3). The resulting capsules were dried at 105° C for 24 hours, and then measure weights. The paraffin content in the capsules was calculated from the weight of the initial paraffins and compared with the result 3.
(4) Stability test of encapsulated PCM
The stability of the encapsulated PCM was examined using a cycle test device. The temperature inside the test tube containing the encapsulated PCM was measured by increasing and decreasing the temperature of the test tube containing the encapsulated PCM from 5°C to 40°C.
In the cycle test, the change in temperature was tested 500 times.
3. Results
3.1 Emulsifier selection
The results of stability tests using Emulsol-P and a polyethylene-based polymer emulsifier at a constant ratio are shown in Fig. 2 In the stability test, it was confirmed that non-encapsulated paraffin was formed from the beginning of the encapsulation reaction when the oil layer was formed. The type and amount of emulsifier were determined through the experiments and the emulsion stability test results were in good agreement with the results of the actual polymerization experiments (Fig. 2)15). Fig. 1(a) presented the poor encapsulation distribution without MMA addition whereas the best diffusion was observed in Fig. 2(d).
3.2 First encapsulation method
(1) Emulsification condition
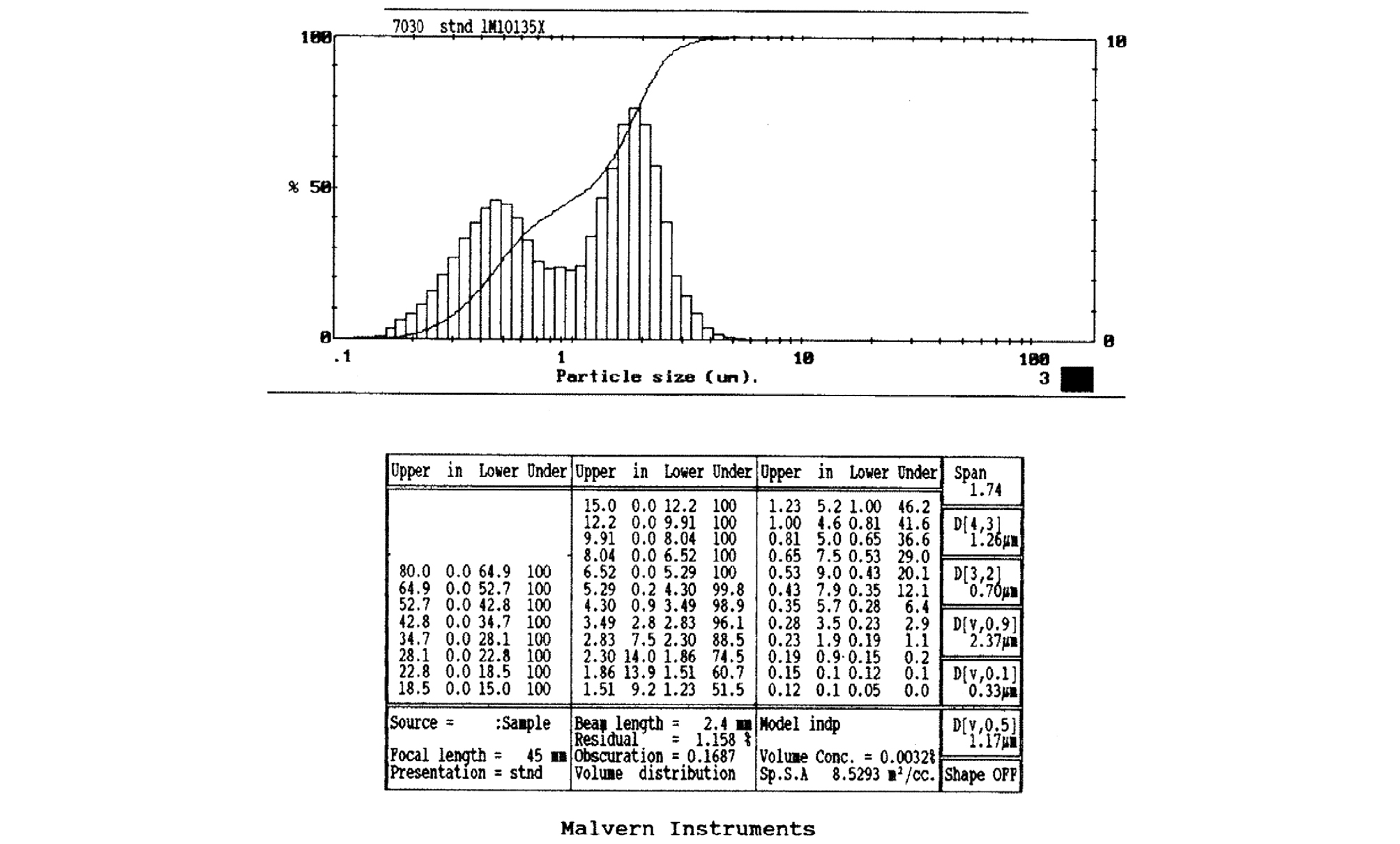
Stirring speed during emulsification: The effect of homogenizer for high speed stirring and general stirrer was investigated in emulsifying organic phase such as PCM. When the homogenizer was used for high - speed agitation, an emulsion with a diameter of 0.2 ~ 0.3 µm was obtained and its size was relatively uniform. On the other hand, when the emulsion was stirred at 300 rpm using a general stirrer, a relatively large emulsion with 1 µm particles were obtained. It was confirmed that the size of the emulsion obtained after polymerization was controlled according to the degree of stirring at the time of emulsification.
(2) Impact of initiators
In the case of using sodium peroxydisulfate as an initiator, the force is applied to the obtained powder, the oil component is felt and the strength of the encapsulated is weak. In addition, when ammonium peroxydisulfate is used as an initiator, the powder form seems to be in a fairy fine state, but the strength seems to be no different from the case of sodium peroxydisulfate. However, for the case of sodium hydrogen sulfate monohydrate, during the polymerization, it seems to have been polymerized in the agitator blade and a lot of agglomerates are formed.
As the result of examining the effect of the initiator concentration on the monomer contrast, the initiator concentration did not affect the polymerization time. However, the stable polymerization could be achieved when the initiator concentration was 0.5 wt%. It was demonstrated that the physical properties of the final encapsulated product were greatly influenced by the type of the initiator. The conversion of initiator at 0.5 wt% was 65 ~ 70% after 1 hour, 78 ~ 83% after 2 hours and 85 ~ 90% after 3 hours. It was confirmed that the conversion rate of the first reaction can act as a reference for starting the secondary encapsulation, and the content of PCM in the microcapsules obtained after the completion of the reaction is also greatly influenced.
(3) The ratio of PCM to monomer
The results showed that stable capsule was obtained up to 67% of PCM ratio. If the ratio of PCM and monomer was 3 : 1 or more, the obtained capsule was unstable. That is, after drying, the capsules were aggregated or hardly broken. The size of the capsules was relatively uniform from 0.2 to 0.3 µm (Fig. 3). The capsules obtained by encapsulating with only the acrylic monomer are low thermal and mechanical properties. In order to supplement them, a method of secondary encapsulation with a monomer which can obtain excellent thermal and physical properties was required.
(4) Polymerization temperature
When the polymerization was carried out at 60°C, the degree of polymerization was very low even when the polymerization was carried out for 20 hours or more, indicating that no encapsulation was achieved. Increasing the polymerization temperature above 75°C could achieve the encapsulation. The higher the temperature, the shorter the polymerization time.
(5) Polymerization rate
From the above results, it is important to control the polymerization rate so that the polymerization rate does not become too high in the encapsulation of PCM. It is more important to control the polymerization rate by controlling the polymerization temperature or changing the amount of the initiator. In this study, the ratio of the amount of encapsulated PCM to the amount of charged PCM was used to calculate the encapsulation efficiency and it was 99.9%.
The microcapsules of paraffin obtained by first encapsulation was presented in Fig. 3
3.3 Second Encapsulation
(1) Determination of secondary monomer input
The content of paraffin in the final capsule is determined by the amount of first and secondary encapsulating wall materials (acrylic and melamine), the degree of polymerization and the timing of secondary encapsulation. That is, the conversion rate of each polymer varies according to the polymerization time in the first encapsulation and affects the weight of the wall material at the second encapsulation16). Thus, the content of paraffin in the final obtained microcapsule is controlled by adjusting the first and second encapsulation polymerization. It was confirmed that if the amount of first and secondary monomers was smaller, the content of paraffin was increased due to the lower content of the wall material after the final encapsulated monomer injection time. In addition, the lower the amount of the melamine as the secondary monomer, the lower the thermal and physical stability of the particles. Therefore, the lower the amount of the input is not desirable.
(2) Drying method
The physical properties of the capsules according to the drying method were confirmed. Drying was performed by spray drying, freezing drying, and drying in a thermostat, and the appearance of the obtained capsule powder was compared. As a result, the heat and PCM encapsulation efficiencies did not show a significant difference in all three cases.
However, when freeze drying was used, the state of the dried particles was the softest. This is because, when freeze-drying is used, the secondary particles that can be formed during drying due to drying at a low temperature are small, thus the size of secondary particles formed by gathering first particles is relatively small. Powder dried in a thermostat was not visually large, and there was no significant difference in softness. In commercial mass production, drying in a thermostat was selected as the most appropriate method because it is fast and most economical.
3.4 Physical properties of encapsulated particles
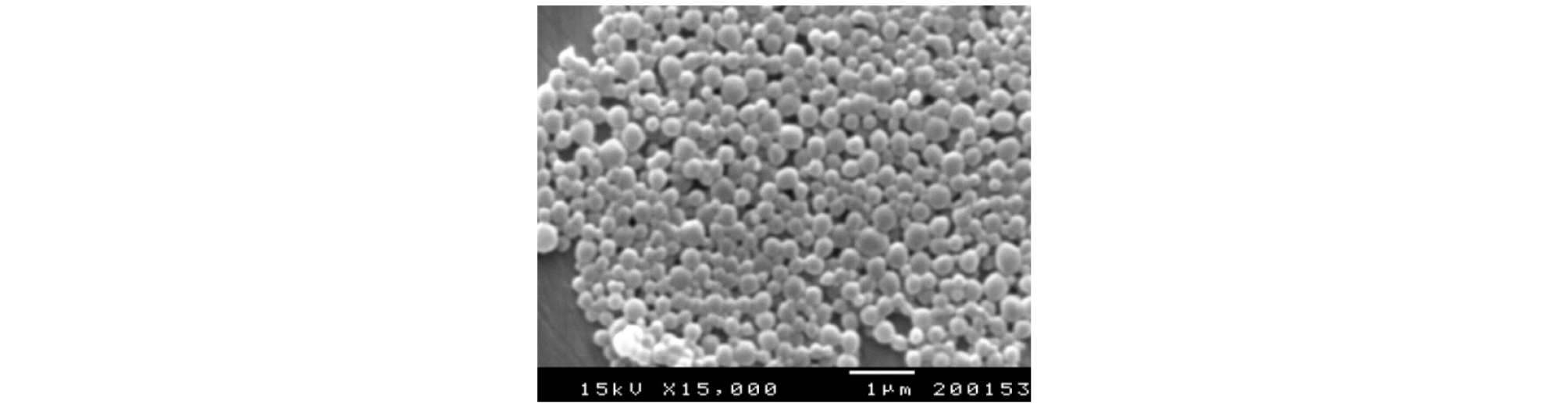
(1) Particle Size
The particle size and size distribution of the particles were observed through a scanning electron microscope. The microcapsules of paraffin obtained by double-encapsulation was presented in Fig. 4 representative particles are shown in Fig. 5 The size of the first particles was almost the same as that of capsules prepared by the first encapsulation method, and it was confirmed that the first particles were three-dimensionally bonded to form secondary grains like clusters. This is considered to be the result obtained by polymerizing the melamine and formaldehyde monomers used in the secondary capsules and producing bonds with neighboring monomers. The size of the particles varied depending on the emulsification method and emulsifier concentration, but it was found that the first particles were about 0.2 ~ 0.3 µm and formed secondary particles of about 3 ~ 10 µm (Figs. 4, 5).
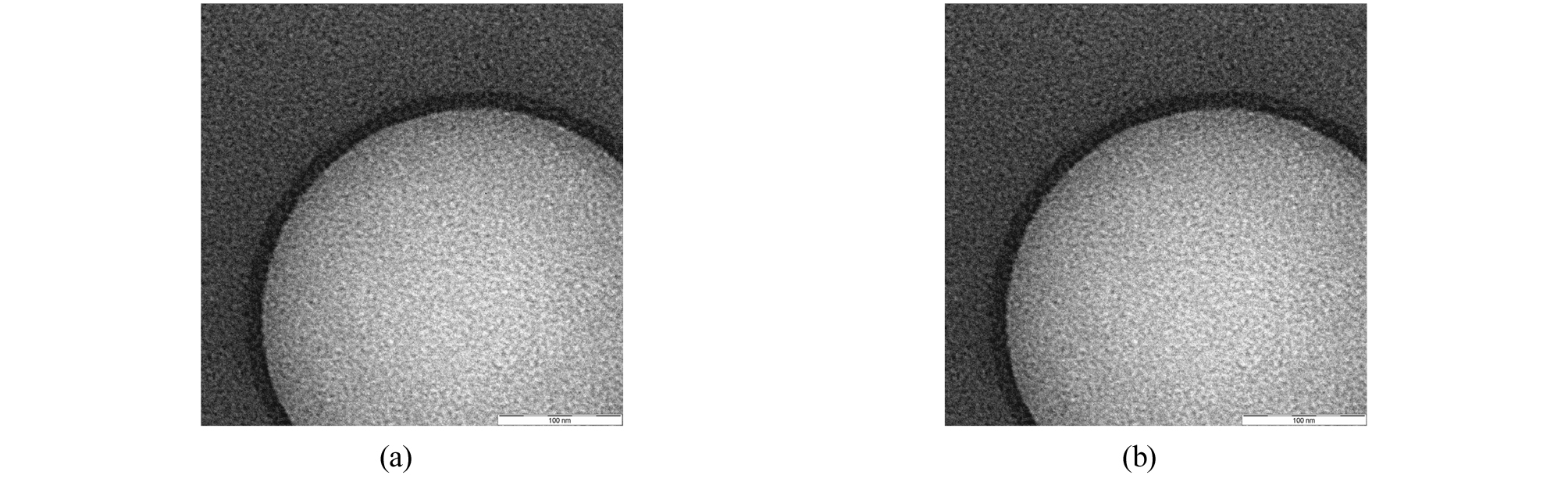
(2) Identification of double capsules
In the case of particles encapsulated solely with a single wall material, the boundary layer could not be confirmed in a uniform wall material. On the other hand, in the case of a double-walled material, a double layered boundary layer could be identified (Figs. 3, 4). The particle size of single wall and double wall was about 0.3 µm (Fig. 6(a), 6(b)).
(3) Encapsulation efficiency
The encapsulation efficiency of paraffin was determined by comparing the initial weight of paraffin wax with the amount of unencapsulated paraffin suspended in the upper part after polymerization and measuring the dry weight. However, the results of this study show that the amount of unencapsulated paraffin was very small or not, as long as the amount of emulsifier was very small or the polymerization temperature did not deviate greatly from the optimum temperature (< 0.1%) when using the double encapsulation method.
(4) Stability test of encapsulated PCM
In the cycle test, the change in temperature was tested 500 times, but there was no change in latent heat according to the number of repetitions. Based on this result, there will be no problem in the stability of the encapsulated PCM.
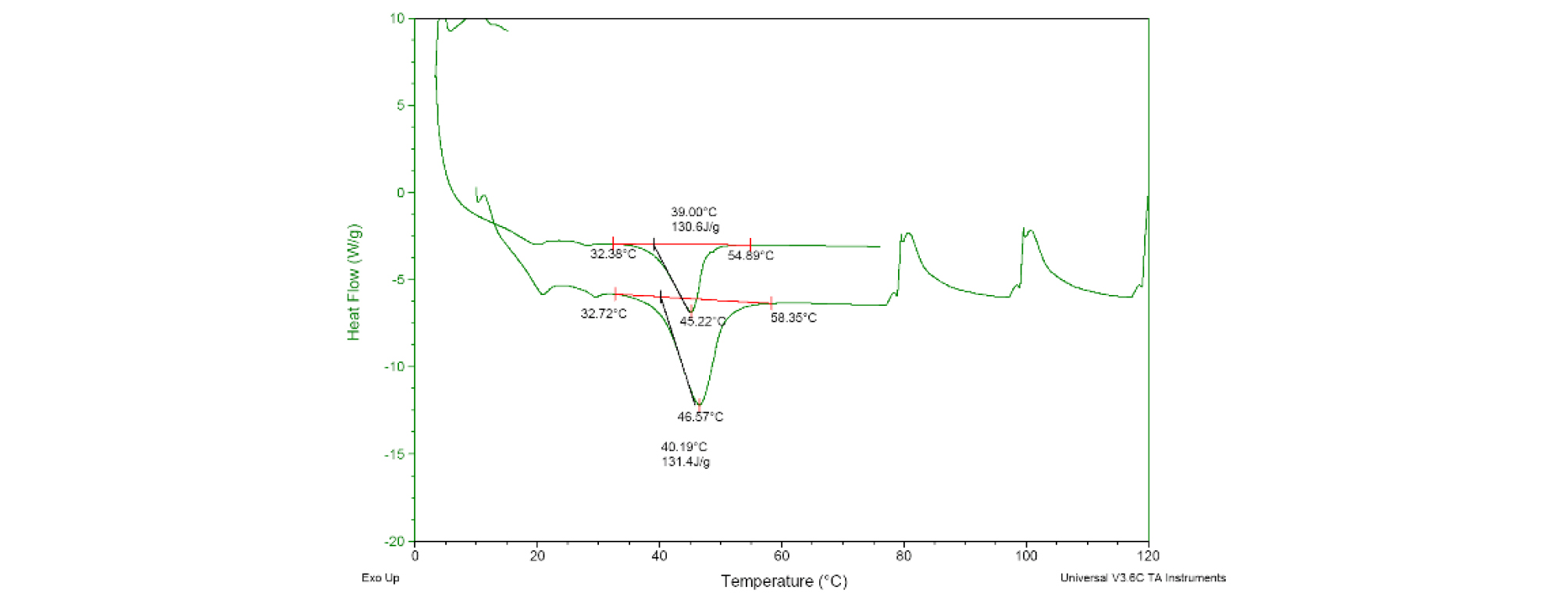
The stability of the encapsulated paraffin was confirmed by two consecutive measurements through DSC, and the results of the endothermic amount are shown in Fig. 7 The two peaks were slightly different (45.2°C and 46.6°C), but the latent heat values were 130.6 and 131.4 J/g.
4. Discussion
In emulsification process, the PCM and MMA monomers may be mixed and dispersed in aqueous phase because they have good solubility with each other. Alternatively, MMA should be added after first PCM is dispersed. It allows to polymerize and encapsulate MMA outside the PCM. When the PCM was mixed with the monomer and dispersed beforehand, relatively good capsules could be obtained. In other case, when the monomer was added separately and polymerized, the encapsulation was greatly lowered. In the former case, the reaction rate is relatively slow, and the monomer in the organic phase migrates to the aqueous phase interface during encapsulating. In the latter case, the emulsion of only monomer is formed and the monomer used to surround the PCM is reduced.
Depending on the amount of PCM per unit of encapsulated particles, the capacity of latent heat storage will vary. If the ratio of PCM in the particles increases, the latent heat storage capacity per unit mass of the particles increases, and when it decreases, the latent heat storage capacity decreases accordingly. Therefore, ratio of PCM to polymer is closely related to latent heat storage capacity, and it is important to optimize PCM ratio. If ratio of PCM in the particles is very high, the PCM may not be completely encapsulated, or the stability of the capsule may be affected13). When the PCM is encapsulated with a monomer, the thickness of the capsule is determined according to the ratio between the two substances. Assuming that the PCM and monomer are 3: 1 and the monomer uniformly surrounds the PCM outside the capsule, the thickness of the capsule is only about 10% of the particle radius. That is, if the diameter of the particles is 1 μm, the thickness is only about 0.05 μm, and the smaller the diameter of the particles, the smaller the thickness of the particles.
The size of the first particles is already determined in the process of encapsulating paraffins with acrylic polymers. The formation of secondary clusters is mainly formed by bonding with neighboring capsules during secondary encapsulation using melamine during polymerization, and also by drying. That is, if the content of melamine was lowered, the polymerization rate was lowered, and the drying temperature was lowered, the formation of secondary particles could be minimized and the size of secondary particles could be made smaller. Thus, the size of capsules was determined by first encapsulation polymerization using acrylic polymer, and submicron-sized paraffin microcapsules with thermal improvement and physical properties were obtained by using a melamine polymer as a secondary encapsulating wall material17). It was confirmed that the particle size was determined in the process of encapsulating with the acrylic polymer as the first wall material, and the physical and thermal stability was imparted by encapsulating with the secondary wall material melamine. From DSC result, the stability of the encapsulated paraffin was confirmed to be very high.
5. Conclusion
The first encapsulation using acrylic polymer and the secondary encapsulation using melamine polymer have been developed in this study. Since emulsifier is consider as the most important factors in the encapsulation process, the stability test was conduct to select the appropriate emulsifier. In the first encapsulation process, emulsification condition, initiator, ratio of PCM to monomer and polymer temperature were investigated to obtain the high efficiency capsules. Sodium hydrogen sulfate monohydrate seems to be a good initiator in this study. The ratio of PCM and monomer is 2:1 and the temperature above 75°C is the appropriate condition for encapsulating. The secondary polymerization conditions such as amount of initiator, content of paraffin, temperature, and the amount of secondary polymer and injection time were determined. The capsules obtained by the encapsulation using MMA exhibited a diameter of 0.2 to 0.3 µm. It was confirmed that thermal and physical properties were improved when secondary encapsulation was carried out by introducing a melamine polymer. As a result of the encapsulation of paraffin by the double encapsulation method, it was confirmed that the particle size was determined in the process of encapsulating using the acrylic polymer as the first wall material, and the physical and thermal stability were imparted using the secondary wall material melamine. In the future, this encapsulated PCM could be applied in many fields such as textile, paint, adhesive sheet, ect.